1 Answer Nathan L. Jun 7, 2017 2.30 ×1024 atoms Ca Explanation: We’re asked to calculate the number of atoms of Ca in 153 g Ca. What we must first do is convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g mol ): 153 g Ca( 1mol Ca 40.08g Ca) = 3.82 mol Ca
Encoding of the colorectal cancer metabolic program through MICU2 | bioRxiv
answered • expert verified How many atoms are in 121 g of calcium? Advertisement Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 aliasger2709 The elements are composed of atoms that have definite mass and atomic number. The number of atoms can be estimated by Avogadro’s number, there are 1.81 × 10²⁴ Ca atoms.

Source Image: quora.com
Download Image
About this tutor ›. Avogadro’s number = 6.02×10 23 particles per mol (particles can be atoms, molecules, formula units, etc) atomic mass Ca = 40.08 g / mol. moles Ca = 3456 g x 1 mol / 40.08 g = 86.228 moles. atoms of Ca = 82.228 moles x 6.02×10 23 atoms / mol = 519.1×10 23 atoms = 5.191×10 25 atoms of Ca. ANSWER (a)

Source Image: brainly.in
Download Image
Materials | Free Full-Text | Impact of the Atomic Packing Density on the Properties of Nitrogen-Rich Calcium Silicate Oxynitride Glasses Sep 8, 2023Step 2: Calculate the number of moles of calcium in 143 g. Number of moles = mass / molar mass Number of moles = 143 g / 40.08 g/mol Number of moles = 3.567 mol Answer Step 3: Calculate the number of atoms in 3.567 moles of calcium. Avogadro’s number states that there are 6.022 x 10^23 atoms in one mole of any substance.

Source Image: pubs.acs.org
Download Image
How Many Atoms Are In 123 G Of Calcium
Sep 8, 2023Step 2: Calculate the number of moles of calcium in 143 g. Number of moles = mass / molar mass Number of moles = 143 g / 40.08 g/mol Number of moles = 3.567 mol Answer Step 3: Calculate the number of atoms in 3.567 moles of calcium. Avogadro’s number states that there are 6.022 x 10^23 atoms in one mole of any substance. Therefore, 143 grams of calcium contain: N = 143 g × 6.022 × 1 0 23 m o l − 1 40.08 g m o l = 2.15 × 1 0 24 \beginaligned N&=\frac\pu143 g \times 6.022 \times 10^23 mol^-1\pu40.08 \fracgmol\\ &=\pu2.15 \times 10^24 \endaligned N = 40.08 mol g 143 g × 6.022 × 1 0 23 mo l − 1 = 2.15 × 1 0 24 2.15 × \times × 10 24
The Atomic-Level Structure of Cementitious Calcium Aluminate Silicate Hydrate | Journal of the American Chemical Society
Chemistry Chemistry questions and answers How many atoms are in 123 g of calcium? atoms This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: How many atoms are in 123 g of calcium? atoms Show transcribed image text There are 2 steps to solve this one. SOLVED: Determine the number of atoms in 61.0 grams of calcium, Ca: (The mass of one mole of calcium is 40.08 g.)

Source Image: numerade.com
Download Image
Solved The formula for calcium phosphate is Ca3(PO4)2. Weigh | Chegg.com Chemistry Chemistry questions and answers How many atoms are in 123 g of calcium? atoms This problem has been solved! You’ll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer Question: How many atoms are in 123 g of calcium? atoms Show transcribed image text There are 2 steps to solve this one.

Source Image: chegg.com
Download Image
Encoding of the colorectal cancer metabolic program through MICU2 | bioRxiv 1 Answer Nathan L. Jun 7, 2017 2.30 ×1024 atoms Ca Explanation: We’re asked to calculate the number of atoms of Ca in 153 g Ca. What we must first do is convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g mol ): 153 g Ca( 1mol Ca 40.08g Ca) = 3.82 mol Ca

Source Image: biorxiv.org
Download Image
Materials | Free Full-Text | Impact of the Atomic Packing Density on the Properties of Nitrogen-Rich Calcium Silicate Oxynitride Glasses About this tutor ›. Avogadro’s number = 6.02×10 23 particles per mol (particles can be atoms, molecules, formula units, etc) atomic mass Ca = 40.08 g / mol. moles Ca = 3456 g x 1 mol / 40.08 g = 86.228 moles. atoms of Ca = 82.228 moles x 6.02×10 23 atoms / mol = 519.1×10 23 atoms = 5.191×10 25 atoms of Ca. ANSWER (a)

Source Image: mdpi.com
Download Image
Short-Range Structure of Amorphous Calcium Hydrogen Phosphate | Crystal Growth & Design CHM • CHM 151L • Rated Helpful Question Answered step-by-step Asked by ProfessorCloverTurtle72 2. How many atoms are in 123 g of calcium? 3. If there are 5.90 moles of O, how many moles of each of the compounds are present? a. H2SO4 b. C2H4O2 c. NaOH 4. The density of phenol, C6H6O, is 1.07 g/mL.

Source Image: pubs.acs.org
Download Image
Nutrients | Free Full-Text | Magnesium Status and Calcium/Magnesium Ratios in a Series of Cystic Fibrosis Patients Sep 8, 2023Step 2: Calculate the number of moles of calcium in 143 g. Number of moles = mass / molar mass Number of moles = 143 g / 40.08 g/mol Number of moles = 3.567 mol Answer Step 3: Calculate the number of atoms in 3.567 moles of calcium. Avogadro’s number states that there are 6.022 x 10^23 atoms in one mole of any substance.

Source Image: mdpi.com
Download Image
CHEMICAL QUANTITIES Composition Stoichiometry Calculating Molar Mass Avogadro’s Number and the Mole Percentage Composition and Empirical Formulas Molecular. – ppt download Therefore, 143 grams of calcium contain: N = 143 g × 6.022 × 1 0 23 m o l − 1 40.08 g m o l = 2.15 × 1 0 24 \beginaligned N&=\frac\pu143 g \times 6.022 \times 10^23 mol^-1\pu40.08 \fracgmol\\ &=\pu2.15 \times 10^24 \endaligned N = 40.08 mol g 143 g × 6.022 × 1 0 23 mo l − 1 = 2.15 × 1 0 24 2.15 × \times × 10 24
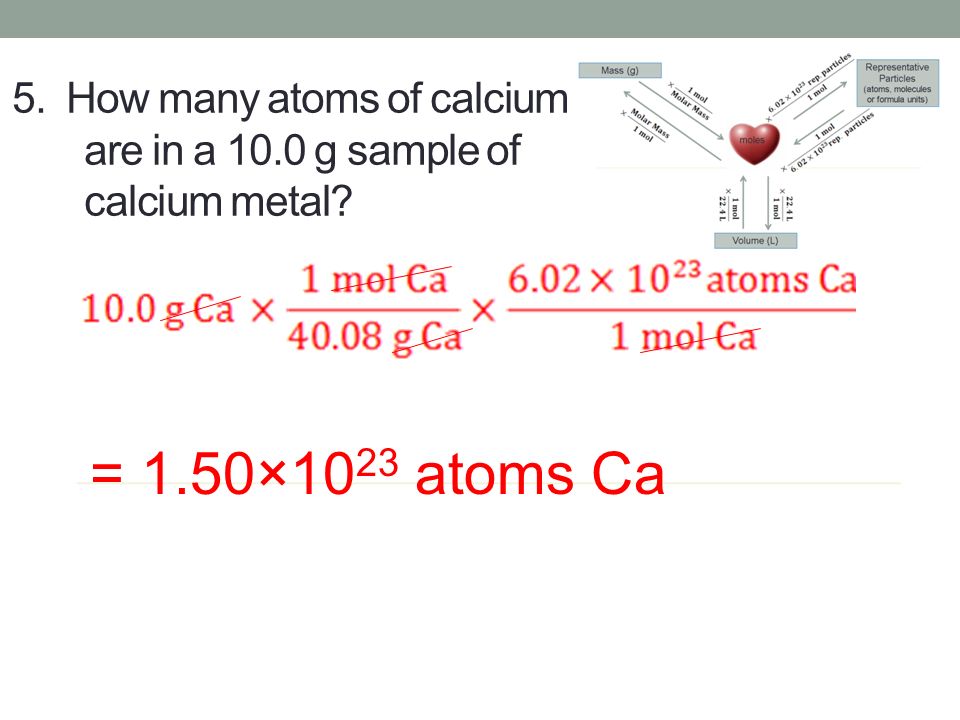
Source Image: slideplayer.com
Download Image
Solved The formula for calcium phosphate is Ca3(PO4)2. Weigh | Chegg.com
CHEMICAL QUANTITIES Composition Stoichiometry Calculating Molar Mass Avogadro’s Number and the Mole Percentage Composition and Empirical Formulas Molecular. – ppt download answered • expert verified How many atoms are in 121 g of calcium? Advertisement Expert-Verified Answer question No one rated this answer yet — why not be the first? 😎 aliasger2709 The elements are composed of atoms that have definite mass and atomic number. The number of atoms can be estimated by Avogadro’s number, there are 1.81 × 10²⁴ Ca atoms.
Materials | Free Full-Text | Impact of the Atomic Packing Density on the Properties of Nitrogen-Rich Calcium Silicate Oxynitride Glasses Nutrients | Free Full-Text | Magnesium Status and Calcium/Magnesium Ratios in a Series of Cystic Fibrosis Patients CHM • CHM 151L • Rated Helpful Question Answered step-by-step Asked by ProfessorCloverTurtle72 2. How many atoms are in 123 g of calcium? 3. If there are 5.90 moles of O, how many moles of each of the compounds are present? a. H2SO4 b. C2H4O2 c. NaOH 4. The density of phenol, C6H6O, is 1.07 g/mL.