Sep 22, 2022How to Determine the Charge of an Ion, Element, and Atom Examples and Practice Problems Conquer Chemistry 26.1K subscribers Subscribe Subscribed 170 23K views 1 year ago 🎯 Want to ace
What is the charge of an atom in its elemental form? – Quora
n = 1.00 C × 1 proton 1.602 × 10 − 19 C = 6.25 × 10 18 protons. 18.1. The same number of electrons is required to make −1.00 C of electric charge. The fundamental unit of charge is often represented as e. Thus, the charge on a proton is e, and the charge on an electron is − e. Mathematically, e = + 1.602 × 10 −19 C.

Source Image: study.com
Download Image
Sep 21, 2023This chemistry video tutorial explains how to determine the charge of an element in it’s ionic form.Chemistry – Basic Introduction:

Source Image: byjus.com
Download Image
How can we find the number of protons and electrons of any element? | Socratic
Determine the formal charge for each atom in NCl 3. Answer: N: 0; all three Cl atoms: 0. Using Formal Charge to Predict Molecular Structure. The arrangement of atoms in a molecule or ion is called its molecular structure. In many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure

Source Image: brainly.com
Download Image
How To Determine Charge Of An Atom
Determine the formal charge for each atom in NCl 3. Answer: N: 0; all three Cl atoms: 0. Using Formal Charge to Predict Molecular Structure. The arrangement of atoms in a molecule or ion is called its molecular structure. In many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure
Jul 27, 2022To calculate the formal charge of an atom, use the equation below. Take the valence number of the atom and subtract the number of bonds and the number of non-bonding electrons. … Hint: Instead of calculating the formal charge for every atom in a molecule, it is a lot faster to identify the atoms that deviate from the “typical” bonding
Determine the formal charge on each atom in the structure. – brainly.com
We can calculate an atom’s formal charge using the equation FC = VE – [LPE – ½ (BE)], where VE = the number of valence electrons on the free atom, LPE = the number of lone pair electrons on the atom in the molecule, and BE = the number of bonding (shared) electrons around the atom in the molecule. Created by Sal Khan. Questions Tips & Thanks
Nucleus of Atom: Composition, Structure, Size & Properties

Source Image: geeksforgeeks.org
Download Image
Your Step by Step Guide to Find Valence Electrons -praxilabs
We can calculate an atom’s formal charge using the equation FC = VE – [LPE – ½ (BE)], where VE = the number of valence electrons on the free atom, LPE = the number of lone pair electrons on the atom in the molecule, and BE = the number of bonding (shared) electrons around the atom in the molecule. Created by Sal Khan. Questions Tips & Thanks
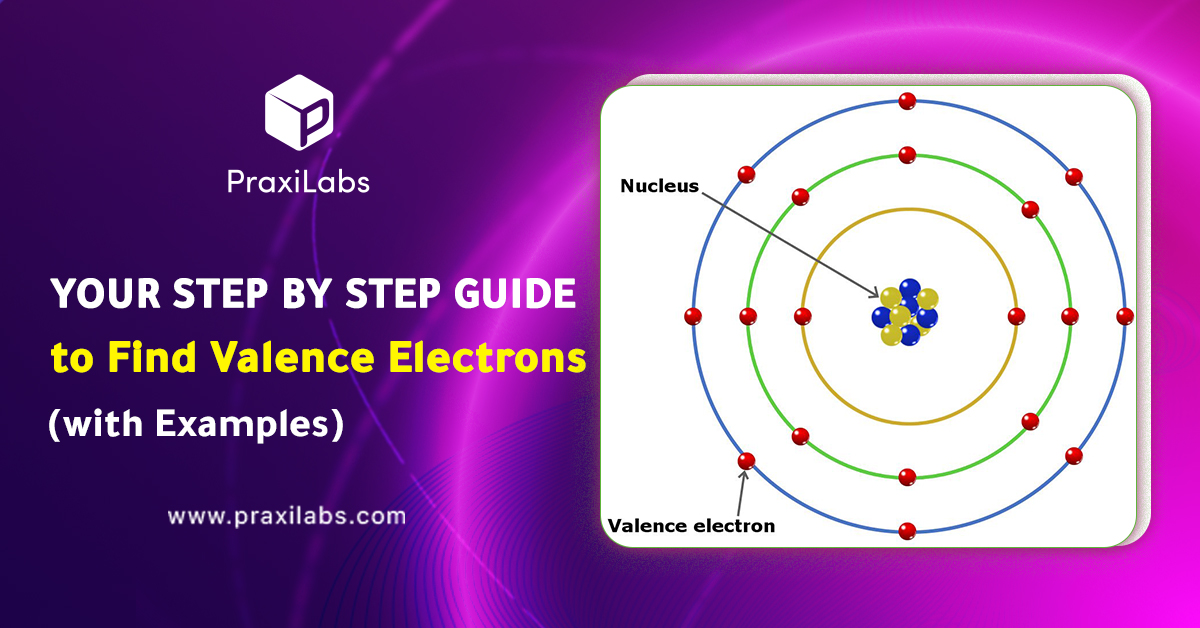
Source Image: praxilabs.com
Download Image
What is the charge of an atom in its elemental form? – Quora
Sep 22, 2022How to Determine the Charge of an Ion, Element, and Atom Examples and Practice Problems Conquer Chemistry 26.1K subscribers Subscribe Subscribed 170 23K views 1 year ago 🎯 Want to ace
Source Image: quora.com
Download Image
How can we find the number of protons and electrons of any element? | Socratic
Sep 21, 2023This chemistry video tutorial explains how to determine the charge of an element in it’s ionic form.Chemistry – Basic Introduction:

Source Image: socratic.org
Download Image
How many resonance structures does N3- have? | Socratic
Apr 29, 2023Understanding the formula The formula for calculating atomic charge is quite simple: AC = P – E Where: AC is the Atomic Charge (eV) P is the number of protons E is the number of electrons See also Sodium Hypochlorite Pool Shock Calculator Online To calculate the atomic charge, subtract the number of electrons from the number of protons.
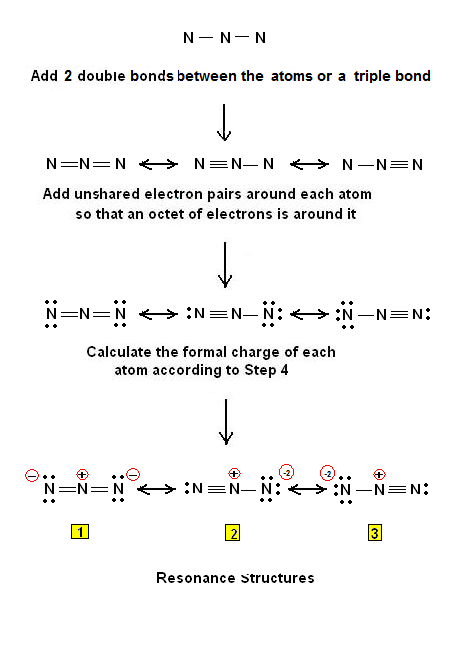
Source Image: socratic.org
Download Image
Chemistry Net: Simple Procedure for writing Lewis Structures- CO2, NCO- – Examples #2
Determine the formal charge for each atom in NCl 3. Answer: N: 0; all three Cl atoms: 0. Using Formal Charge to Predict Molecular Structure. The arrangement of atoms in a molecule or ion is called its molecular structure. In many cases, following the steps for writing Lewis structures may lead to more than one possible molecular structure
Source Image: chem-net.blogspot.com
Download Image
electronique: Zener Diode
Jul 27, 2022To calculate the formal charge of an atom, use the equation below. Take the valence number of the atom and subtract the number of bonds and the number of non-bonding electrons. … Hint: Instead of calculating the formal charge for every atom in a molecule, it is a lot faster to identify the atoms that deviate from the “typical” bonding
Source Image: electronique1.blogspot.com
Download Image
Your Step by Step Guide to Find Valence Electrons -praxilabs
electronique: Zener Diode
n = 1.00 C × 1 proton 1.602 × 10 − 19 C = 6.25 × 10 18 protons. 18.1. The same number of electrons is required to make −1.00 C of electric charge. The fundamental unit of charge is often represented as e. Thus, the charge on a proton is e, and the charge on an electron is − e. Mathematically, e = + 1.602 × 10 −19 C.
How can we find the number of protons and electrons of any element? | Socratic Chemistry Net: Simple Procedure for writing Lewis Structures- CO2, NCO- – Examples #2
Apr 29, 2023Understanding the formula The formula for calculating atomic charge is quite simple: AC = P – E Where: AC is the Atomic Charge (eV) P is the number of protons E is the number of electrons See also Sodium Hypochlorite Pool Shock Calculator Online To calculate the atomic charge, subtract the number of electrons from the number of protons.