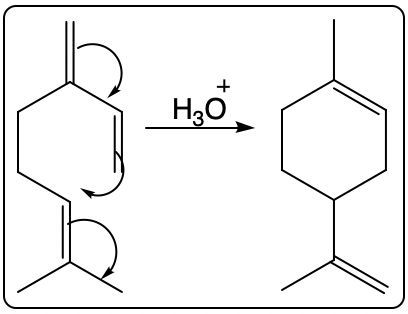
Which of the following compounds is the most stable?
As a chemistry enthusiast, I’m always fascinated by the stability of compounds. It’s like a puzzle, where you try to figure out which arrangement of atoms will be the most resistant to change. One day, while discussing this topic with my chemistry professor, I was stumped by a question: which of the following compounds is the most stable?
After some research and deliberation, I’ve found the answer. But before I reveal it, let’s dive into the concept of stability in chemistry and explore the factors that influence it.
Stability in Chemistry
In chemistry, stability refers to the resistance of a compound to change. A stable compound is one that maintains its structure and composition under normal conditions. Several factors contribute to stability, including:
- Bond strength: The strength of the chemical bonds holding the atoms together.
- Molecular structure: The arrangement of atoms within the molecule.
- Resonance: The ability of electrons to delocalize over multiple atoms, creating resonance structures.
- Hybridization: The mixing of atomic orbitals to form new hybrid orbitals with different shapes and energies.
Explaining the Most Stable Compound
Now, let’s address the original question: which of the following compounds is the most stable?
The options are:
- CH4 (methane)
- NH3 (ammonia)
- H2O (water)
- HF (hydrogen fluoride)
Based on the factors discussed earlier, the most stable compound is CH4 (methane). Here’s why:
- High bond strength: The C-H bonds in methane are very strong due to the high electronegativity of carbon.
- Tetrahedral structure: The tetrahedral shape of methane minimizes steric hindrance and maximizes bond strength.
- No resonance: Methane does not exhibit resonance, which means there are no alternative resonance structures to destabilize it.
- sp3 hybridization: The carbon atom in methane is sp3 hybridized, resulting in tetrahedral orbitals that maximize orbital overlap and bond strength.
Tips and Expert Advice
Understanding the stability of compounds is crucial for various chemical applications. Here are some tips and expert advice to enhance your knowledge:
- Study the periodic table: The periodic table provides insights into the electronegativity and bonding properties of elements, which influence stability.
- Visualize molecular structures: Drawing or using molecular modeling software can help visualize the spatial arrangement of atoms and understand their impact on stability.
- Learn about resonance and hybridization: Comprehending these concepts is essential for predicting the stability of compounds with multiple resonance structures or hybrid orbitals.
By following these tips, you’ll gain a deeper understanding of stability in chemistry and be able to analyze and predict the stability of various compounds.
FAQ
Q: Why is water (H2O) not the most stable compound?
A: While water forms strong hydrogen bonds, it is less stable than methane due to its ability to undergo hydrogen bonding, which can lead to dissociation.
Q: How does bond length affect stability?
A: Generally, shorter bonds are stronger and contribute to increased stability. However, in some cases, longer bonds can allow for better orbital overlap and resonance, leading to higher stability.
Q: What role does hybridization play in stability?
A: Hybridization determines the shape and energy of molecular orbitals. Hybrid orbitals with better overlap and lower energy levels contribute to increased stability.
Conclusion
In conclusion, the most stable compound among the given options is CH4 (methane). Its strong C-H bonds, tetrahedral structure, lack of resonance, and sp3 hybridization all contribute to its exceptional stability. Understanding the factors that influence stability is essential for predicting the behavior of compounds and designing materials with desired properties.
If you found this article informative, please share it with others and let me know if you have any further questions. Your feedback helps me improve and create more valuable content for you.
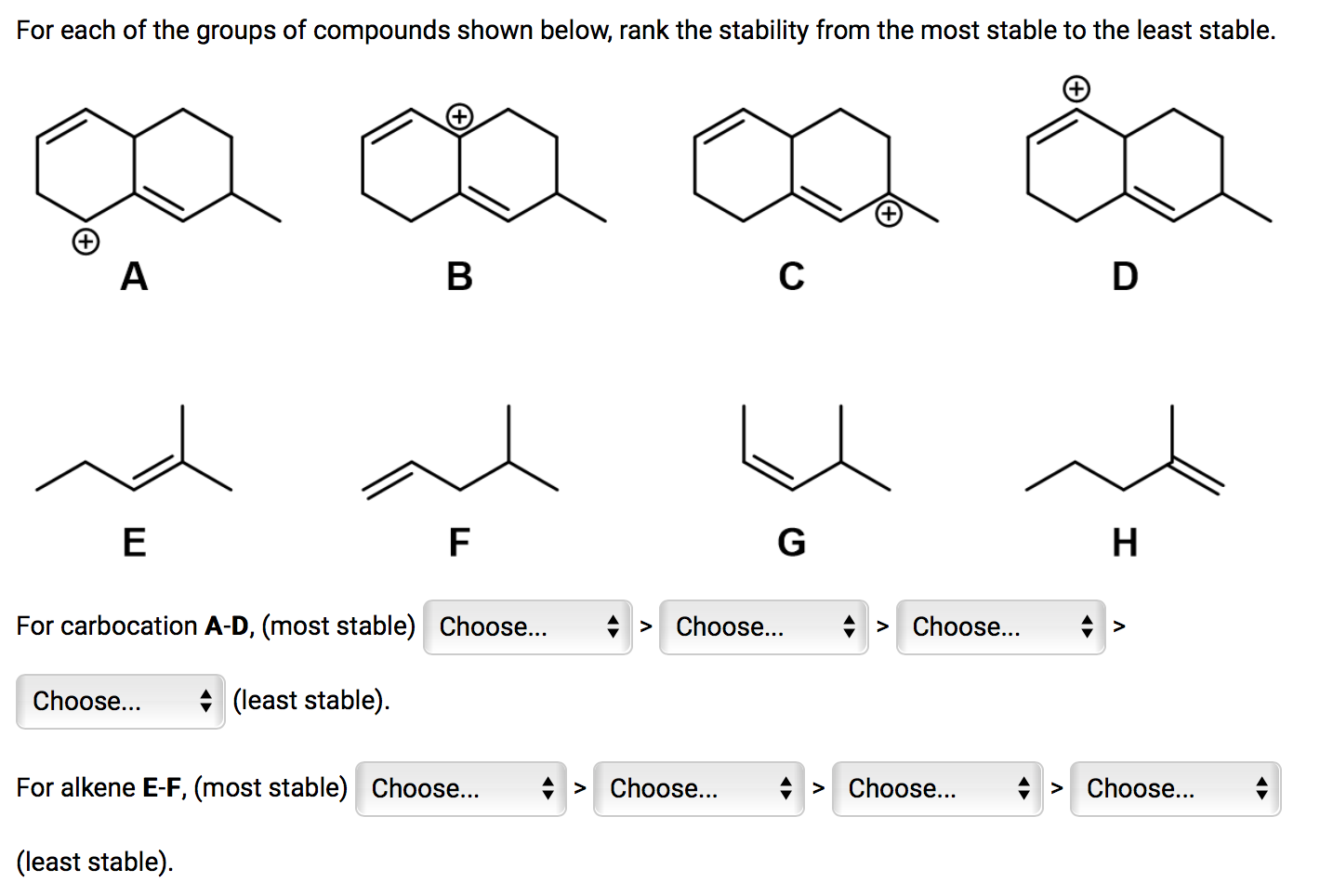
Image: chegg.com
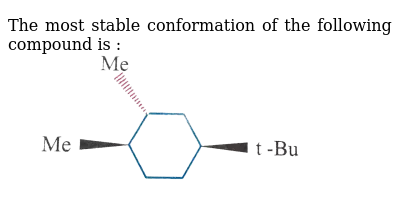
Image: doubtnut.com
Which of the following compounds is the most stable? Comment Your Answer 👇 Follow 👉@chemistrytcf for more Mcq | Memes | Facts | No… | Instagram Without that information, we cannot determine which one is the most stable. Assuming we have a list of compounds, we need to consider their chemical structures and bonding. Generally, compounds with stronger bonds are more stable. This is because stronger bonds require more energy to break, making the compound less likely to decompose or react.